CASE OF THE WEEK
2020-19 / May 11
(Contributor: Stephanie J Conrad and Ming Zhou)
A 53 year old male with pT3b prostate cancer developed gross hematuria 20 months after receiving external beam radiation. A cystoscopy showed friable mucosa, which was thought to be suspicious for bladder cancer.
Quiz
1. What is the correct diagnosis?
a. Nested Variant Urothelial Carcinoma (NVUC)
b. Florid von Brunn Nests
c. Inverted Papilloma
d. Inverted Urothelial Carcinoma
e. Pseudocarcinomatous Epithelial Hyperplasia
2. What immunohistochemical marker with diffuse staining is most useful to support the diagnosis?
a. CD44
b. CK 20
c. p53
d. p63
e. HMW keratin
3. What histological feature supports the diagnosis?
a. Hyperplastic inverted urothelial nests
b. Rare mitoses
c. Irregular urothelial nests associated with infiltrating single cells
d. Urothelial nests encircling vessels with fibrin deposits
e. Invasion of the detrusor muscle
1. e
2. a
3. d
1. Pseudocarcinomatous Epithelial Hyperplasia
2. CD44
3. Urothelial nests encircling vessels with fibrin deposits
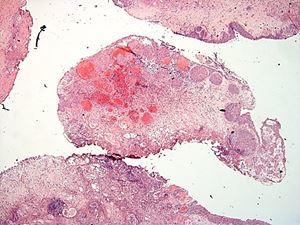
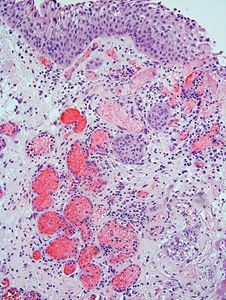
The lesion consists of circumscribed proliferative urothelial nests within and limited to the lamina propria, with some nests exhibiting a central squamoid appearance. There is mild nuclear atypia, however, the nuclear to chromatin ratio is low and mitoses are rare. There is extensive vascular ectasia with fibrin deposits in some vascular spaces. The proliferating epithelial nests surround the ectatic vessels. There are also mixed inflammatory cells in the stroma.
The morphological pattern seen in this case is so-called “pseudocarcinomatous epithelial hyperplasia”, which can be seen in a number of reactive conditions, including radiation cystitis (most commonly), intravesical chemotherapy, severe peripheral vasculopathy, or in the presence of an indwelling catheter. These conditions result in local ischemia and epithelial hyperplasia as the result. When “pseudocarcinomatous epithelial hyperplasia” is considered, one should seek the patient’s history for radiation to the pelvis or intravesical treatment.
Radiation exposure increases the risk for the development of urothelial carcinoma. However, it appears pseudocarcinomatous hyperplasia is a benign, reactive process, and its presence is not associated with an increased risk of development of urothelial carcinoma.
The differential diagnosis is broad and includes florid Von Brun nests, inverted papilloma, nested variant urothelial carcinoma (NVUC) and invasive high grade urothelial carcinoma with inverted growth pattern (1, 2). Florid von Brunn nests exhibit large solid nests, with regular spacing between them. Cystitis cystica et glandularis is often seen. Inverted papillomas have nests of peripheral palisading basal cells, and lack reactive stroma. NVUC is composed of numerous urothelial nests of variable sizes. Single cells and fused nests are commonly seen. The base of the lesion is ragged and infiltrative and the tumor may invade muscularis propria. Invasive high grade urothelial carcinoma with inverted growth demonstrates infiltrative growth border. Although pseudocarcinomatous hyperplasia may mimic invasive carcinoma with infiltrative urothelial nests, the urothelial nests in pseudocarcinomatous hyperplasia are circumscribed, do not invade the muscularis propria, and are present in the background of stromal edema, hemorrhage, hyalinization, ectatic and thrombosed blood vessels, as well as hemosiderin laden macrophages.
An immunohistochemical staining panel of CD44, CK20 and p53 can be used to differentiate pseudocarcinomatous hyperplasia from urothelial carcinoma. Reactive lesions, such as pseudocarcinomatous epithelial hyperplasia, are diffusely positive for CD44. CK20 stains the superficial umbrella cell layer. P53 shows wild type staining pattern with focal and weak staining in the basal layer. In contrast, in urothelial carcinoma, CK20 and p53 stain diffusely the entire epithelium and CD44 is negative (3).
1. Chan TY and Epstein JI. Radiation or Chemotherapy Cystitis with “Pseduocarcinomatous” Features. Am J Clin Pathol. 2004 July; 28:909-913.
2. Wu A. Pseudocarcinomatous Hyperplasia of the Urinary Bladder. Arch Pathol Lab Med. 2014 October; 138: 1268-71.
3. McKenney JK, Desai S, Cohen C, Amin MB. Discriminatory Immunohistochemical Staining of Urothelial Carcinoma in Situ and Non-Neoplastic Urothelium: An Analysis of Cytokeratin 20, p53, and CD44 antigens. Am J Surg Pathol. 2001 Aug; 25(8):1074-8.
Stephanie J Conrad and Ming Zhou
Tufts Medical Center
Boston, MA
sconrad@tuftsmedicalcenter.org
mzhou3@tuftsmedicalcenter.org
Bladder
Pseudocarcinomatous Hyperplasia; Urothelial nests; Fibrin